Events
US Patent Office Issues Allowance for APOSEC Patent
On Sept. 6, 2019, the United States Patent and Trademark Office issued a Notice of Allowance concerning the US patent application No. 13/140,120 “Pharmaceutical preparation”, the patent of APOSEC for the treatment of internal conditions. Aposcience AG expects the patent soon to be granted.
Paper on content analysis of APOSEC published
Beer L et al. Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration – was recently published in Scientific Reports. Sci Rep. 2015 Nov 16;5:16662. doi: 10.1038/srep16662.
PDF Download
Kickoff Meeting for Marsyas I Study
On 23 Oct. 2014 the clinical phase I trial Marsyas I, a prospective, single-Center, randomised, double-blinded, placebo controlled, dose finding phase I study in the indication skin wound healing was initiated. The Kickoff Meeting took place in the Dept. of Clinical Pharmacology of the Medical University of Vienna.
AGES Approval for allogeneic and autologous APOSEC production
On 25 April 2014 the Austrian Health Authority AGES issued the approval for the production of allogeneic and autologous APOSEC for clinical testing in the APOSEC GMP production facilty at the Blood Bank Linz.
Effect of APOSEC on chronic ischemic left ventricular dysfunction
Long-acting beneficial effect of percutaneously intramyocardially delivered secretome of apoptotic peripheral blood cells on porcine chronic ischemic left ventricular dysfunction
Biomaterials. 2014 Jan 16. pii: S0142-9612(13)01567-6. doi: 10.1016/j.biomaterials.2013.12.071. [Epub ahead of print]
Pavo N1, Zimmermann M, Pils D3, Mildner M, Petrási Z, Petneházy Ö, Fuzik J, Jakab A, Gabriel C, Sipos W, Maurer G, Gyöngyösi M, Ankersmit HJ
Abstract
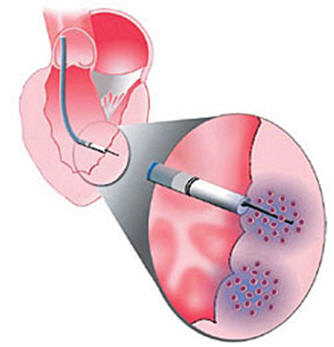
Background. The quantity of cells with paracrine effects for use in myocardial regeneration therapy is limited. Treatment with APOSEC may be more feasible and efficacious. This study investigated the effects of catheter-based endomyocardial injection of APOSEC (secretome of 2.5×109 apoptotic peripheral blood mononuclear cells) on porcine chronic post-myocardial infarction (MI) left ventricular (LV) dysfunction and on gene expression.
Methods. Closed-chest reperfused MI was induced in pigs by 90-min occlusion followed by reperfusion of the mid-LAD (day 0). At day 30, animals were randomized to receive porcine APOSEC (n=8) or medium solution (control; n=8) injected intramyocardially into the MI border zone using 3D NOGA guidance. At day 60, cardiac MRI with late enhancement and diagnostic NOGA (myocardial viability) was performed.Gene expression profiling of the infarct core, border zone, and normal myocardium was performed using microarray analysis and confirmed by quantitative real-time PCR.
Results. Injection of APOSEC significantly decreased infarct size (p<0.05) and improved cardiac index and myocardial viability compared to control. A trend towards higher LV ejection fraction was observed in APOSEC vs. control (45.4±5.9% vs. 37.4±8.9%, p=0.052). Transcriptome analysis revealed significant downregulation of caspase-1, tumor necrosis factor and other inflammatory genes in APOSEC affected areas. PCR showed higher expression of myogenic factor Mefc2 (p<0.05) and downregulated caspase-3 genes (p<0.05) in APOSEC-treated pigs.
Conclusions. Overexpression of MEF2c and repression of caspase-3 was related to decreased infarct size and improved cardiac function in secretome-treated animals. Altered gene expression 1-month post-APOSEC treatment suggested the long-acting effects of injected paracrine factors.
Keywords: cell-free regeneration therapy, genes, myocardial infarction, ischemia, remodeling
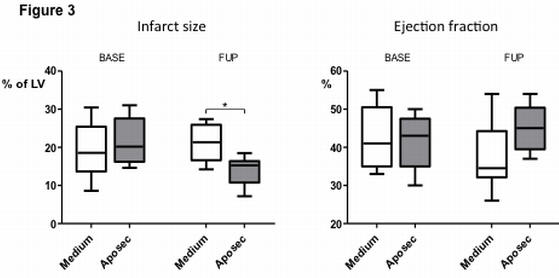
Part of Figure 3 is shown here. Ejection fraction (EF) at day 3 (BASE) and at day 60 (follow-up; FUP) showing a significant increase in EF at FUP in the APOSEC-treated pigs (n=8) compared to the control pigs (n=8). The APOSEC-treated pigs showed significantly smaller infarct size and better cardiac indexes. There was significantly lower end-diastolic pressure and end-diastolic stiffness in the APOSEC-treated group.
*p<0.05
Presentation at the Congress of the European Society of Cardiology, 31 Aug. – 4 Sept. 2013, Amsterdam
Overexpression of GATA4, TGF-β3, MEF2C and HIF1-α contributes to improvement of infarct size in porcine model of chronic myocardial infarction, treated with percutaneous intramyocardial delivery of secretome of apoptotic white blood cells (APOSEC)
P. Noemi, M. Zimmermann, D. Pils, G. Maurer, M. Gyöngyösi, H.J. Ankersmit – Medical University of Vienna – Vienna – Austria
Background: We have investigated the effect of catheter-based endomyocardial injections of APOSEC (secretome of apoptotic peripheral white blood cells) on porcine chronic post-infarction (MI) left ventricular (LV) dysfunction and gene expression profile compared with placebo.
Methods and results: Closed-chest reperfused MI was induced by 90-min occlusion followed by reperfusion of the mid LAD (day 0). At day 30 the animals were randomized and received either porcine APOSEC (n=8) or placebo Medium (n=8) injected into the border zone of MI using 3D NOGA percutaneous intramyocardial guidance. At day 60 tissue samples were collected. Gene expression profiling of the infarct core, border zone and normal myocardium was performed using microarray analysis, confirmed by quantitative real-time PCR. Gene array revealed significant dowregulation of caspase-1 and other inflammatory genes in the APOSEC-affected areas. PCR showed higher levels of expression of myogenic factor Mefc2 (p<0.05) with downregulated caspase-3 (p<0.05) in the APOSEC-pigs.
Conclusions: Overexpression of MEF2c and repression of caspase is related to decrease in infarct size and improved cardiac function in secretome-treated animals. The altered gene-expression 1-month post APOSEC-treatment suggests a long-acting effect of injected paracrine factors.
European Patent Granted to APOSCIENCE AG
Patent for Treatment of Skin Wounds Using Supernatant of PBMCs Granted by the European Patent Office
We are happy to announce that a European Patent for the treatment of skin wounds using supernatant of Peripheral Blood Mononuclear Cells (PBMCs) was granted to APOSCIENCE AG by the European Patent Office.
Patent No.: 2379085
Date of the decision: 13 June 2013
Stem Cell Therapy – When? How?
Forthcoming event:
Tuesday, 07 May 2013, 19:00
Wissenschaftliche Sitzung/Scientific Session
Gesellschaft der Ärzte in Wien – Billrothhaus, Frankgasse 8, 1090 Wien
Sabine Steiner (Dept. of Internal Medicine II, Medical University of Vienna)
Helmut Sinzinger (Dept of Nuclear Medicine, Medical University of Vienna)
Die Prognose von Patienten mit kardiovaskulären Erkrankungen hat sich durch medikamentöse und interventionelle Therapien deutlich verbessert. Für weitere Fortschritte sind neue, innovative Behandlungsansätze notwendig. Die Stammzellentherapie gilt seit Jahren als vielversprechende Option, doch ist sie trotz positiver Proof-of-Concept Studien noch nicht zur klinischen Routine geworden. In dieser Sitzung soll beleuchtet werden, warum dies so ist und wo die Zukunft dieser Therapieform liegen kann.
Speakers:
Massimiliano Gnecchi (University of Pavia):
From cells to cell products:
the fascinating journey of stem cell therapy for heart disease
Mariann Gyöngyösi (Medical University of Vienna):
Stem cell reasearch – Cell less therapy in chronic myocardial infarction
Hendrik Jan Ankersmit (Medical University Vienna)
Prof. Pischingers Leukozytolyse und APOSEC – eine Zeitreise
(Prof. Pischinger’s leukocytolysis and APOSEC – a travel through time)
Cardiovascular Research Symposium
24 January 2013
Cardiovascular Research Symposium,
organized by Prof. Margarethe Geiger, Department of Vascular Biology and Thrombosis Research of the Medical University of Vienna, together with the Christian Doppler Laboratory for Diagnosis & Regeneration of Cardiac and Thoracic Diseases, 24 January 2013, Jugendstilhörsaal of the Medical University of Vienna.